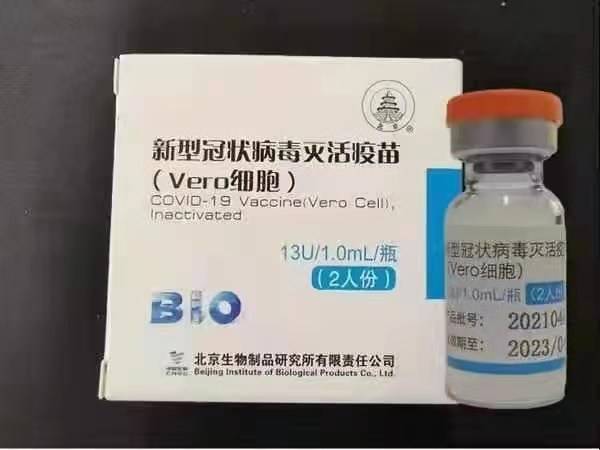
Sinopharm (Beijing): BBIBP-CorV
KANAWAI 1
1 Hoʻokolokolo
ʻO ChiCTR2000032459
Kina
PHASE 2
2 Nā hoʻāʻo
NCT04962906
Alekina
ʻO ChiCTR2000032459
Kina
KANAWAI 3
6 mau hoʻāʻo
NCT04984408
ʻO ChiCTR2000034780
Aupuni 'Emira' Alapia Hui Pū 'ia
NCT04612972
Pelū
NCT04510207
Bahrain, ʻAigupita, Ioredane, United Arab Emirates
NCT04560881, BIBP2020003AR
Alekina
NCT04917523
Aupuni 'Emira' Alapia Hui Pū 'ia
Aponoia
ʻO ka papa inoa hoʻohana ʻōnaehana ʻo WHO 59 Aupuni
Angola 、 Argentina 、 Bahrain 、 Bangladesh 、 Belarus 、 Belize 、 Bolivia (Plurinational State of) 、 Brazil 、 Brunei Darussalam 、 Kambodia 、 Cameroon 、 Chad 、 China 、 Comoros 、 ʻAigupita 、 ʻIrana (Lepupalika ʻIlamika o) 、 ʻIraka 、 Ioredane 、 ʻO Kyrgyzstan 、 Lāpana Lepupalika Kemokalaka o Lao
Lebanona 、 Malaysia 、 Maldives 、 Mauritania 、 auritius 、 Mongolia 、 Montenegro 、 Morocco 、 Mozambique 、 Namibia 、 Nepal 、 Niger 、 North Makedoa 、 Pakistan 、 Paraguay 、 Peru 、 Philippines 、 Repubalika o Kongo Nā Mokupuni 、 Somalia 、 Sri Lanka 、 Thailand 、 Trinidad a me Tobago 、 Tunisia 、 United Arab Emirates 、 Venezuela (Repubalika Bolivarian o) 、 Vietnam Nam 、 Zimbabwe
ʻO Sinopharm BBIBP-CorV COVID-19 kahi lāʻau lapaʻau i hana ʻole ʻia i hana ʻia mai nā ʻāpana virus i ulu i ka moʻomeheu i nele i ka hiki ʻole o ka pathogenic. Ua hoʻomohala ʻia kēia moho lāʻau hoʻomaʻamaʻa e Sinopharm Holdings a me ka Beijing Institute of Biological Products.
Hana ka lāʻau hoʻomaʻamaʻa Sinopharm BBIBP-CorV ma ka ʻae ʻana i ka ʻōnaehana pale e hana i nā mea pale e kūʻē iā SARS-CoV-2 beta coronavirus. Ua hoʻohana ʻia nā lāʻau hoʻomaʻemaʻe inactivated i loko o nā makahiki he mau makahiki, e like me ka lāʻau koʻokoʻo rabies a me ka lāʻau hoʻomaʻi hepatitis A. Ua hoʻohana kūleʻa ʻia kēia ʻenehana hoʻomohala i nā lāʻau lapaʻau kaulana kaulana, e like me ka lāʻau koʻokoʻo rabies.
Ua hoʻokaʻawale ʻia ʻo Sinopharm's SARS-CoV-2 (WIV04 strain and library number MN996528) mai kahi mea maʻi ma ka Haukapila ʻo Jinyintan ma Wuhan, Kina. Hoʻolahalaha ʻia ka maʻi ma ka moʻomeheu i ka laina kelepaʻi kūpono o Vero, a ua hana ʻole ʻia ka supernatant o nā hunaola i hoʻopili ʻia me β-propiolactone (1: 4000 vol / vol, 2 a 8 ° C) no 48 mau hola. Ma hope o ka wehewehe ʻana o ka ʻōpala a me ka ultrafiltration, ua hana ʻia kahi hana β-propiolactone lua ma lalo o nā kūlana like me ka hana ʻole mua. Wahi a WHO, ua hoʻolaha ʻia ka lāʻau lapaʻau ma luna o ka 0.5 mg o ka alum a hoʻoili ʻia i loko o nā syringes i hoʻopiha ʻia i 0.5 mL o ka saline paʻakai paʻakai paʻakai ʻole me nā preservatives ʻole.
Ma Kēkēmapa 31, 2020, ua hoʻolaha aku ka mokuʻāina ʻoihana lāʻau i ka ʻae ʻia o ka lāʻau lapaʻau hoʻokolohua i hoʻomohala ʻia e Sinopharm.
Ma Mei 7, 2021, hoʻolaha ka World Health Organization i ka ʻae ʻia o ka lāʻau āpau. ʻO ka papa inoa hoʻohana hoʻopilikia o WHO i hiki ai i nā ʻāina ke hoʻolalelale i kā lākou mau ʻae hoʻokele ponoʻī e lawe mai a lawelawe i ka lāʻau lapaʻau COVID-19. ʻO ka hui aʻoaʻo aʻoaʻo a WHO e pili ana i nā hoʻolālā Immunization ua hoʻopau pū kekahi i kāna loiloi o ka lāʻau āpau. Ma muli o nā hōʻike āpau i loaʻa, ua paipai ʻo WHO i ʻelua mau lāʻau o ka lāʻau lapaʻau, ʻekolu a ʻehā mau pule ke kaʻawale, no nā mākua he 18 mau makahiki a ʻoi paha. Hoʻohālikelike ʻia ka hopena Vaccine e kūʻē i nā hōʻailona a me nā maʻi maʻi i 79% no nā hui makahiki āpau.
Ua paʻi ka ʻAhahui Lapaʻau ʻAmelika "A Randomized Clinical Trial: Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults" on May 26, 2021, pening that "in this prespecified interim analysis of a randomised clinical trial, nā mākua ʻO nā lāʻau lapaʻau SARS-CoV-2 i hoʻopau ʻole ʻia i lawelawe ʻia i loko o kēia kālailai wā pōkole o nā hoʻokolohua hoʻokolohua kaulike e hoʻoliʻiliʻi i ka makaʻi o ka COVID-19 hōʻailona, a kākaʻikahi nā hanana kūpilikiʻi koʻikoʻi. " I kēia pae 3 hoʻokolohua kaulike ʻia i nā mākua, ka hopena o nā lāʻau āpau āpau i hoʻopau ʻole ʻia i nā maʻi COVID-19 e like me 72.8% a me 78.1%. Loaʻa nā hanana kūpilikiʻi koʻikoʻi he 2 i nā lāʻau lapaʻau like ʻole me ke alapine e like me ka pūʻulu hoʻomalu wale nō, a ʻo ka hapa nui i pili ʻole i ka lāʻau lapaʻau. Ua ʻike ʻia kahi hoʻokolohua noiʻi i nā lāʻau āpau 2 i hoʻokomo ʻia i nā mea pale pale kūlike ʻole e like me nā hopena o ka hoʻāʻo 1/2.
Ua hoʻolaha ka WHO SAGE Working Group i kahi loiloi o ka lāʻau laʻau Sinopharm / BBIBP COVID-19 ma Mei 10, 2021. Hoʻohui ka lāʻau lapaʻau ʻo GAVI's COVID-19 i kahi monitor vial vaccine e haʻi nei i nā limahana olakino inā ua mālama pono ʻia ka lāʻau lapaʻau a ʻaʻole i hōʻike ʻia hoʻomehana wela. A ʻo kahi hopena, hōʻino, ua hōʻike ʻo GAVI ma Mei 14, 2021. nā lepili akamai i hana ʻia e Zebra Technologies a hana ʻia e Temptime Corporation, i loko o kahi pōʻai me kahi pahu kala māmā i waenakonu, i hana ʻia me kahi kemika kala ʻole e hoʻomohala i nā kala i ka manawa. . Lilo kēia i pōʻeleʻele e hāʻawi i kahi ʻike ʻike o ke kūpale ʻana o ka wela. Ke hōʻike ʻia ka ipu i ka wela ma mua o kona palena mālama kūpono, lilo ka pōʻai i pouli ma mua o ka pōʻai, e hōʻike ana ʻaʻole pono e hoʻohana hou ʻia ka lāʻau lapaʻau.
ʻO ka helu inoa inoa hale waihona lāʻau ʻōpala National BBIBP-CorV COVID-19: DB15807.